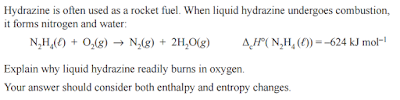Tuesday, 13 September 2016
Friday, 9 September 2016
Equivalence Point pH
STEP ONE: Write a balanced equation for what has happened at the equivalence point. This is not an equilibrium reaction, it is neutralisation.
STEP TWO: Use the n, c, V relationship to calculate the concentration of the conjugate being formed in the neutralisation reaction.
STEP THREE: Write an equation for the dissociation of the conjugate with water.
STEP FOUR: Use the weak base (or weak acid) calculations, as appropriate, to find the concentration of hydronium ions.
STEP FIVE: Use this concentration to calculate pH.
Buffers
The first things we need to master about buffers:
- Define a buffer
- Explain how a buffer works
- Calculate the pH of buffer solutions
On Mondays lesson, we can use our workbooks (Continuing Chemistry), and the internet as our resources, as well as the following videos:
Our next step is to work out how to make a buffer of a desired pH...
Tuesday, 6 September 2016
Titration Curves
During a titration, the pH does not change uniformly. There are key points that can be calculated, and the remainder of the curve can be sketched from these:
Initial pH
This example starts with a weak acid in the conical flask. If it were a weak base, the calculations are a lot harder, but you can refer to THIS for how to do that calculation.
Equivalence Volume and Half-Equivalence Point
This relies on a good memory of the titration topic from last year. Once you know the equivalence volume, you are ready to mark the pH of the half-equivalence volume (pKa = pH).
Final pH
This is very easy if you remember how to calculate the pH of a strong base (in this example). Put all of these points together to sketch the shape of the curve.
Weak Acid vs. Strong Base
Weak Base vs. Strong Acid
Wednesday, 31 August 2016
pH - Weak Bases
Whenever these questions are asked, they are worth Excellence. This is because there are six key steps to remember, and they need to be remembered in order!
- Write out the equation for the base acting as a an alkaline solution.
- Write a Kb expression.
- Calculate Kb from Ka
- Calculate [OH-] from the Kb expression (assuming [HB+] = [OH-]
- Calculate [H3O+] from [OH-], using KW
- Calculate pH
Friday, 26 August 2016
pH - Weak Acids
Weak acids only partially dissociate, so how do we calculate their pH?
We were encouraged to work through pp180-181 in Continuing Chemistry to check whether or not we have understood this.
We were encouraged to work through pp180-181 in Continuing Chemistry to check whether or not we have understood this.
pH - Strong Acids and Bases
This is just a recap of last year, but these skills are vital for moving forward in this topic:
Here is the concept being taught to a Year 12 class:
Thursday, 25 August 2016
Acids, Bases and Salts
We started with a recap of the Bronsted-Lowry definitions of acids, bases and amphiprotic species:
For example:
Then, we looked at the pH of some salts, comparing them to a control of NaCl (known to have a pH of 7.0):
We need to write chemical equations for the ions to justify the observed pH values (alkaline or acidic):
HCO3- + H2O <=> H2CO3 + OH-
This equation shows an increase in [OH-], which is expected as the pH > 7.0
Friday, 19 August 2016
Predicting Precipitation
This was an overview of Ionic Product and we use it to predict if a precipitate will form. Then we looked at what happens when a common ion is added to a saturated solution, for example making a limewater solution more alkaline with sodium hydroxide.
Wednesday, 17 August 2016
Tuesday, 16 August 2016
Friday, 5 August 2016
Electrolysis
This is the last concept we need to learn about in this topic. It is the opposite to the theory of cells in every way except one:
Oxidation occurs at the Anode
Reduction occurs at the Cathode
Friday, 29 July 2016
Electrochemical Cells
Today we made a Zinc-Copper cell and measured its voltage. We then put a group of them in series to amplify the voltage - this is called a battery.
There is a convention for how we write a "cell diagram" for this, and we will explore that more next week.
Standard electrode potentials are measured by setting up the hydrogen half-cell as the anode. The redox pair that you want to know the electrode potential for is then set up as the cathode, and the voltage recorded - this is the electrode potential for this particular redox pair.
Wednesday, 27 July 2016
Wednesday, 6 July 2016
Oxidation-Reduction
Today we recalled the work we had done on: oxidants and reductants; observations and half equations; and oxidation numbers:
Friday, 1 July 2016
Entropy
This video sums it up very nicely:
It is worthwhile knowing the sorts of questions we may be asked about entropy as well:
----------------------
Wednesday, 29 June 2016
Enthalpy Calculations - Enthalpy of Formation and Hess's Law
USING ENTHALPIES OF FORMATION
USING HESS'S LAW
The following is how we could use Hess's Law to solve the same problem as used for showing using Enthalpies of Formation:
Friday, 24 June 2016
Wednesday, 22 June 2016
Molar Heat of Fusion
We did an experiment to find the molar heat of fusion for water, by melting ice and recording temperature changes.
We found our results did not match the theoretical values due to our inability to control certain variables, such as energy transfer with the environment.
We then explored the concept of molar heat of fusion:
We found our results did not match the theoretical values due to our inability to control certain variables, such as energy transfer with the environment.
We then explored the concept of molar heat of fusion:
Students used this template to explore molar heat of vaporisation, next...
Tuesday, 21 June 2016
Energy Recap
The background of the work we are about to get into includes:
COLLISION THEORY
Specifically, we need to be aware that changing temperature has an effect upon the energy of the system. To keep things "fair", we need to compare things at the same temperature (or at a specific temperature).
ENTHALPY CHANGES
One of the key concepts we need to remember from last year is enthalpy change:
Thursday, 16 June 2016
Intermolecular Forces
Today, we had the following tasks:
- Reading pp93-4; 96 in Continuing Chemistry
- Graphng task pp95-6 in Continuing Chemistry
- Homework sheets (Lewis structures etc.)
Friday, 10 June 2016
Wednesday, 8 June 2016
Lewis Structures and Shapes - Level 2 Recap
We need to remember how to draw Lewis Dot Diagrams:
Then, we need to understand the shapes that molecules (and polyatomic ions) can be:
Once we have these mastered, we can look at Lewis Structures for polyatomic ions, exceptions to the Octet Rule, and shapes with more 5 and 6 for their Steric Number.
 |
| https://figures.boundless.com/10397/full/vsepr-geometries.png |
Once we have these mastered, we can look at Lewis Structures for polyatomic ions, exceptions to the Octet Rule, and shapes with more 5 and 6 for their Steric Number.
Thursday, 26 May 2016
Periodic Trends
There are a couple of properties of atoms that have characteristic trends. We need to be able to discuss these trends in terms of atomic structure, specifically the forces acting within an atom that affect these properties.
ELECTRONEGATIVITY
1ST IONISATION ENERGY
Subscribe to:
Comments (Atom)